Preparing for Joint Clinical Assessment: The increased importance of Indirect Treatment Comparisons
Background
We are now less than three months away from the introduction of the Joint Clinical Assessment (JCA) for ATMPs and oncology products – representing a significant landscape shift for the European Pharmaceutical sector.
This new centralised JCA process aims to streamline the clinical assessment of health technologies and accelerate patient access to innovative treatments across EU member states. The JCA intends to reduce the duplication of effort currently observed by countries performing independent clinical assessments for new technologies as part of HTA submissions.
However, the JCA presents new challenges for health technology developers (HTDs). Evidence needs must now reflect the requirements of all 27 member states in a single JCA submission. The need to demonstrate comparative efficacy in this joint framework is paramount, and indirect treatment comparisons (ITCs) are set to play a pivotal role in this process.
Why Indirect Treatment Comparisons (ITCs) are critical for the JCA process
Under the JCA process, HTDs must provide evidence that reviews a new technology in the context of existing standards of care, recognising that these may significantly differ across member states. Given that treatments deemed to be standard of care may evolve prior to regulatory approval and that it is infeasible to design clinical trials which address all possible interventions simultaneously, indirect comparisons are required.
Indirect treatment comparisons (ITCs) allow HTDs to compare their technology with potential comparator treatments in the absence of direct H2H trials, by collecting data from alternate sources (e.g. independent clinical trials and real world evidence) and performing statistical analyses to help estimate relative effectiveness of the technology against comparators.
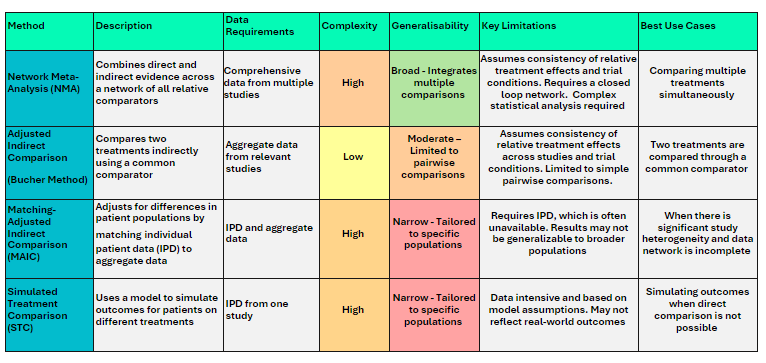
Statistical methods utilised in the conduct of ITCs include network meta-analyses (NMAs), the Bucher method, matching-adjusted indirect comparisons (MAICs) and other approaches such as simulations utilising individual patient data:
A recent analysis by Macabeo et al.i identified that 120/543 (22%) of HTA evaluation reports for oncology treatments in EU4/UK countries from 2018-2021 presented an ITC, with an overall acceptance rate of 30%. Whilst many EU member states currently accept ITCs as part of an considering the multiple policy questions a JCA dossier will need to address, it is clear the role of ITCs will become significantly elevated.
In the context of the JCA, comparators chosen as part of the PICOs (population, intervention, comparator, outcome) submitted by each market can differ significantly depending on local treatment guidelines, accessibility and prescribing patterns. A novel technology will need to demonstrate value not only against the standard of care in one country, but against all comparators listed in the PICOs selected by the rapporteurs representing the HTA CG (HTA Coordination Group). ITCs enable these comparisons of effectiveness to be made in a relatively inexpensive manner.
Given that each member state is obliged to consider the outcome of the JCA process as part of national decision making, the importance of ITCs is paramount. ITCs will become a central component of JCA submissions going forward to support demonstration of comparative efficacy of technologies where directly observed comparative data is lacking.
How health technology developers can prepare for challenges posed by an increased need for ITCs
To address the upcoming challenges associated with an increased need for ITCs, we suggest that HTDs do the following in preparation for a JCA submission:
1. Internal assessment of resource, budget and capability to perform ITCs concurrently in a short timeline:
multiple ITCs will likely be required for each technology’s JCA submission, necessitating statistical expertise, a rapid understanding of requirements, and dedicated time allocated to perform analyses required
anticipating resource needs for JCA dossier preparation and planning ahead is key given the 100 day timeline from date of consolidated PICO lists being provided to HTDs to submission
Decisive Consulting have developed a ‘JCA readiness assessment’ tool to support HTDs to assess, track and plan for JCAs across an organisation
2. Prediction of PICOs for pipeline assets likely to be assessed via JCA
appropriate planning means that ITCs likely to be required for upcoming JCA submissions can be anticipated and performed prior to receiving the final consolidated PICOs
predicting the likely PICOs a technology will encounter as part of the JCA process early on in development will help to optimise pivotal trial design, aligning outcomes with those used for comparators, in order to facilitate feasible ITCs
Decisive Consulting have an AI-enabled process https://www.picopredict.com/ predicting the HTA needs of EU member states, providing an understanding of likely comparators and hence the ITCs required
Written by Milind Sood
Decisive Dialogue 15th October 2024
If you’d like to hear more about how we can support you prepare for JCAs, you can contact enquiries@decisiveconsulting.co.uk – or look out for our stands at WODC and ISPOR!